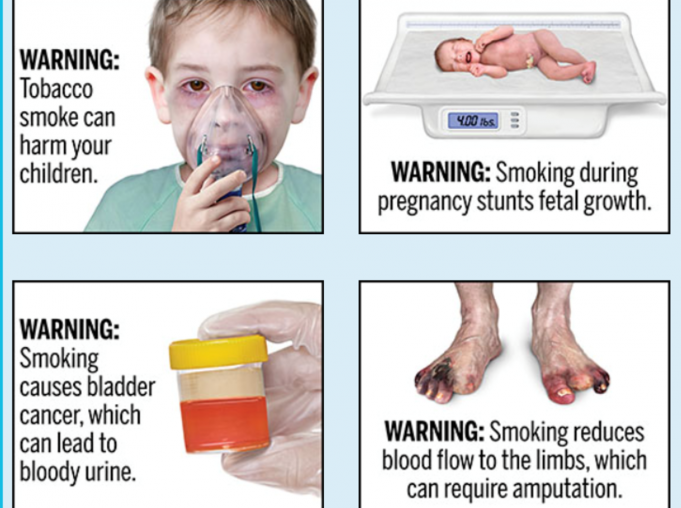
The United States has long been lagging behind other countries with regards to cigarette warnings. In fact, the FDA has not updated cigarette pack warnings for over 30 years, since 1984.
In total there are 11 warnings, featuring such statements that are accompanied by color graphic images of health conditions caused by smoking. Originally set to go into effect on June 18th, 2021, the FDA had already delayed the ‘effective date’ to October 16, 2021.
This delay was the result of two federal lawsuits filed to halt the process. One of those lawsuits, R.J. Reynolds Tobacco Co. et al. v. United States Food and Drug Administration, filed on April 3, was filed by R.J. Reynolds Tobacco Co., and several manufacturers and retailers in the Eastern District of Texas. The objective of the suit was invalidating the FDA’s rule for these new warnings and Congress’s requirement that the FDA mandate them.
FDA asks for health warnings’ rotational plans
In line with the court’s order, the FDA has announced that it strongly encourages the releveant parties to submit cigarette health warnings’ rotational plans as soon as possible, but no later than March 16, 2021, rather than Dec. 16, 2020, as previously required. In addition, the agency plans to revise its relevant guidance documents related to the graphic cigarette health warning regulations with the new effective dates.
Meanwhile the lawsuits unashamedly filed by tobacco companies, namely R.J. Reynolds, Philip Morris USA Inc. and Sherman Group Holdings, LLC., who also keep professing they want to contribute to a smoke-free world, are still ongoing.
Read Further: CSP